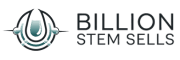The slow erosion of memory and self in Alzheimer’s disease (AD) represents one of modern medicine’s most profound challenges. As we witness loved ones fade into cognitive shadows, the limitations of conventional therapies become painfully evident. Yet emerging research suggests we may be approaching an inflection point, where regenerative medicine offers more than symptomatic relief—potentially rewriting the disease’s trajectory.
Understanding the Alzheimer’s Landscape
Alzheimer’s isn’t merely “forgetfulness.” This neurodegenerative disorder progressively destroys neurons, characterized pathologically by amyloid-beta plaques and neurofibrillary tau tangles. The synaptic chaos manifests through memory disintegration, confusion, personality shifts, and eventual loss of bodily functions. Current FDA-approved medications—acetylcholinesterase inhibitors and NMDA antagonists—provide modest symptomatic delay but fail to halt neurodegeneration. This therapeutic impasse has fueled exploration into regenerative approaches.
hUC-MSCs: More Than Simple Cell Replacement
Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) possess unique biological properties that position them as promising AD therapeutics. Unlike embryonic stem cells, these ethically sourced cells exhibit low immunogenicity and strong immunomodulatory functions. Their therapeutic mechanism extends beyond neuronal replacement:
Paracrine signaling: hUC-MSCs secrete neurotrophic factors (BDNF, GDNF) that rescue damaged neurons and stimulate synaptic regeneration.
Microglial modulation: They suppress neuroinflammation by converting destructive microglia into neuroprotective phenotypes.
Amyloid clearance: Through enhanced phagocytosis and enzymatic degradation (neprilysin upregulation).
Notably, a 2022 Stem Cell Research & Therapy study demonstrated that intravenous hUC-MSCs in AD mice reduced amyloid burden by 40% and improved cognitive scores by selectively homing to inflamed brain regions—a biological “triage” system we’re only beginning to understand.
CRISPR: Precision Engineering for Neurodegeneration
 While stem cells provide biological “hardware,” CRISPR-Cas9 gene editing offers the “software” upgrade. This molecular scalpel allows precise modification of disease-associated genes in stem cells before transplantation. In AD contexts, CRISPR-enhanced approaches include:
While stem cells provide biological “hardware,” CRISPR-Cas9 gene editing offers the “software” upgrade. This molecular scalpel allows precise modification of disease-associated genes in stem cells before transplantation. In AD contexts, CRISPR-enhanced approaches include:
- APOE4 correction: Converting the high-risk ε4 allele to neuroprotective ε3 variants
- BACE1 knockout: Reducing amyloidogenic processing by targeting the beta-secretase gene
- Neurotrophic boosters: Inserting genes for enhanced BDNF or NGF production
A groundbreaking 2023 study in Nature Neuroscience combined CRISPR-edited MSCs with nanoparticle delivery, achieving 68% greater amyloid clearance than unmodified cells in primate models. This synergy exemplifies how engineered cells become targeted drug factories within the brain.
The Gathering Storm—and a Path Forward
With global AD cases projected to triple to 152 million by 2050 (Alzheimer’s Disease International, 2023), the urgency is palpable. Recent clinical developments underscore the potential:
Phase I/II trials (NCT03172117, NCT02600130) show hUC-MSC infusion significantly improves MMSE scores in mild-to-moderate AD patients, with benefits persisting for 12+ months post-treatment. PET imaging reveals reduced amyloid accumulation and preserved metabolic activity in critical memory regions.
Yet challenges remain—optimizing delivery methods, preventing cell senescence, and ensuring long-term safety. The most promising research now focuses on combinatorial approaches: CRISPR-enhanced stem cells delivered via intranasal routes to bypass the blood-brain barrier, potentially coupled with anti-tau biologics.
As we stand at this interdisciplinary crossroads, regenerative strategies offer more than incremental progress. They represent a fundamental shift from managing decline to rebuilding neural integrity. While not yet a panacea, the convergence of stem cell biology and precision gene editing provides tangible hope that we might one day transform Alzheimer’s from a terminal diagnosis to a treatable condition—preserving not just memories, but identities.
References: Alzheimer’s Disease International (2023). World Alzheimer Report. | Kim et al. (2022). Stem Cell Res Ther. 13(1):489. | Liu et al. (2023). Nat Neurosci. 26(5):765-778. | ClinicalTrials.gov identifiers NCT03172117, NCT02600130.