The way stem cells distinguish themselves from other cells is one of the most captivating processes I’ve studied in medical science. These master cells stand out as the body’s only cells that can turn into over 200 different specialized cell types. They’re quite remarkable – unlike regular cells that have limited lifespans, stem cells keep renewing and creating exact copies of themselves.
Stem cells are the building blocks that make up our bodies. Your body uses them as its natural repair system. Hematopoietic stem cells maintain blood and immune cells, while mesenchymal stem cells support bone, cartilage, muscle, and fat. The sort of thing I love about these cells is their talent to become different types of cells – from blood cells to nerve cells and heart muscle cells. This makes them a great way to get insights for medical research and treatment.
Stem cell therapy shows promise for treating diseases of all types. This becomes crucial since about 60% of American adults live with a chronic disease, showing a real need for new treatments. The list keeps growing – from arthritis and multiple sclerosis to various cancers and heart disease. These cells offer hope when regular treatments don’t work.
This piece will get into the science of cellular reprogramming and how scientists utilize stem cells to change medicine. We’ll look at what drives cells to develop, the quickest ways to guide cell growth, and the exciting clinical uses that are improving patient care.
Molecular Basis of Stem Cell Differentiation
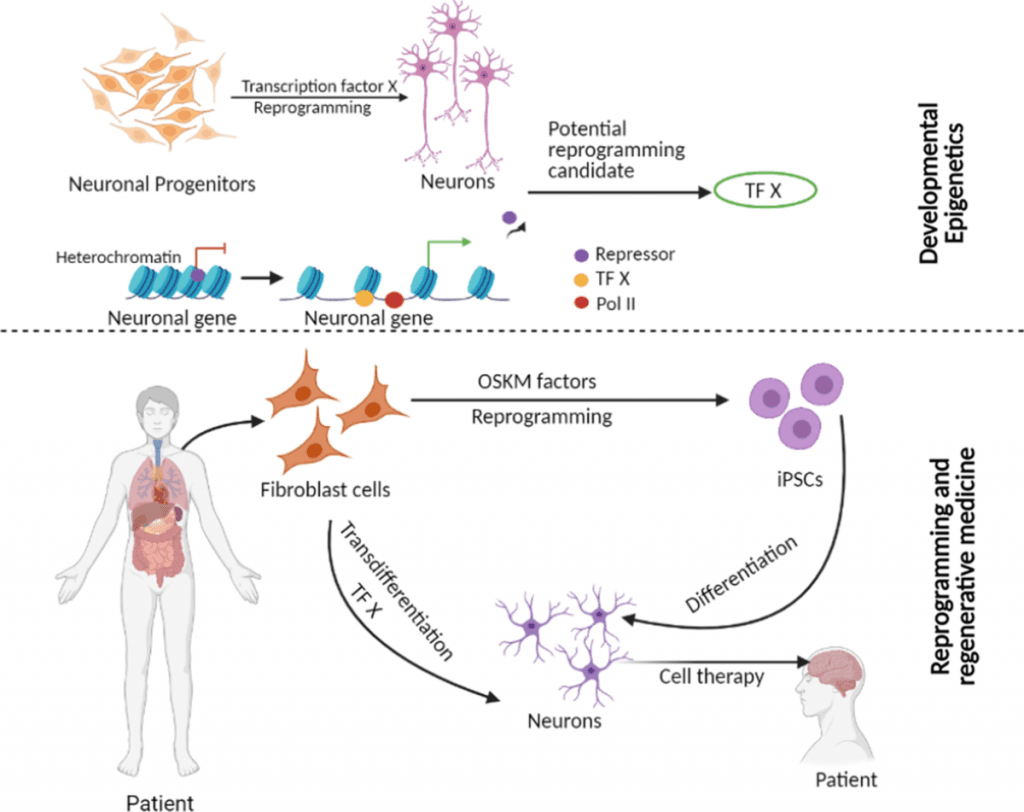
Image Source: Clinical Epigenetics – BioMed Central
The molecular complexity of stem cell separation depends on detailed genetic and epigenetic mechanisms that change unspecialized cells into functional, tissue-specific ones. Scientists now see understanding these mechanisms as crucial to advance regenerative medicine approaches.
Pluripotency vs Multipotency: Key Differences
A stem cell’s separation capacity defines its cellular potency. Pluripotent stem cells (PSCs) can separate into cells from all three germ layers—ectoderm, mesoderm, and endoderm. These cells cannot form extraembryonic tissues like placenta or umbilical cord. Multipotent stem cells separate only into closely related cell types within a specific lineage. Blood-forming stem cells (HSCs) produce exclusively blood cell types, and mesenchymal stem cells (MSCs) develop into bone, cartilage, muscle, and fat cells . This fundamental difference in separation potential determines which diseases each stem cell type might treat.
Transcription Factors in Lineage Commitment
Transcription factors (TFs) act as primary regulators during stem cell separation. Core TFs for pluripotent cells include OCT4, SOX2, and NANOG, which maintain self-renewal capabilities. These factors work by binding to specific DNA sequences and control gene expression essential for maintaining stemness.
Different TFs arrange cell fate decisions during lineage commitment. To cite an instance, see:
- RUNX2 and Osterix guide MSCs toward bone formation
- PPARγ directs adipogenesis
- SOX9 controls chondrogenic differentiation
- GATA-1, GATA-2, and PU.1 regulate hematopoietic development
Research has showed that activating even a single lineage-specific TF can convert progenitor cells into different lineages.
Epigenetic Regulation During Differentiation
Epigenetic mechanisms shape stem cell fate significantly beyond transcription factors. DNA methylation, histone modifications, and non-coding RNA regulation form these mechanisms.
Genes linked to self-renewal become progressively silent while lineage-specific genes activate during differentiation. Dynamic changes in DNA methylation patterns at CpG sites and modifications of histone proteins drive this process. Methylation at histone H3K4 activates genes, while methylation at H3K27 silences them . Chromatin-modifying enzymes with opposing activities enable precise and reversible regulation needed for cellular reprogramming.
Cellular Reprogramming and Induced Pluripotent Stem Cells (iPSCs)
Cellular reprogramming revolutionized our grasp of cell fate plasticity and created new possibilities in regenerative medicine. This section will get into how genetic manipulation can revert differentiated cells to a pluripotent state.
Yamanaka Factors and iPSC Generation
Shinya Yamanaka and Kazutoshi Takahashi made a breakthrough in 2006 when they reprogrammed mouse fibroblasts into induced pluripotent stem cells (iPSCs) . They found four key transcription factors—Oct3/4, Sox2, Klf4, and c-Myc—now known as the Yamanaka factors that could turn somatic cells into pluripotent stem cells. Their success with adult human dermal fibroblasts followed in 2007 .
These Yamanaka factors trigger vital developmental signaling pathways that maintain pluripotency. Specifically:
- Oct4 and Sox2 function as core factors in regulating developmental processes
- Klf4 improves Oct4 and Sox2 activities
- c-Myc plays a distinct role in metabolic processes
Somatic Cell Nuclear Transfer vs iPSC Reprogramming
Nuclear reprogramming happens through two main methods: somatic cell nuclear transfer (SCNT) and iPSC generation. SCNT employs an unfertilized egg’s cytoplasm to reprogram a somatic cell genome into a totipotent state. This represents the most complete epigenetic reprogramming process . iPSC technology takes a different approach by resetting somatic cells through temporary overexpression of transcription factors without human eggs.
Both methods can create patient-specific pluripotent stem cells. SCNT works well in various vertebrates but faces technical, legal, and ethical hurdles. iPSC reprogramming proves technically simpler yet remains nowhere near as efficient or quick as SCNT.
Risks of Genetic Instability in Reprogrammed Cells
iPSCs show promise but raise concerns about genomic integrity. Comparative genomic hybridization analysis shows deletions and amplifications in reprogrammed cells. These signatures point to oncogene-induced DNA replication stress. The genomic changes depend by a lot on c-Myc expression.
The process shows low efficiency (about 0.01–0.1%), which suggests problems with the technique. Cells with impaired p53 pathways reprogram more easily when scientists block tumor suppressor genes like p53. Early-passage iPSCs also contain more copy number variants than intermediate-passage cells or fibroblasts.
Materials and Methods: Culturing and Directing Differentiation
Scientists need precise control over cellular environments to implement stem cell differentiation effectively. Research teams have developed more sophisticated methods to culture and guide these versatile cells’ development over the last several years.
Feeder-Free Culture Systems for Pluripotent Cells
Mouse embryonic fibroblasts (MEFs) were the foundation of traditional stem cell culture. These cells secreted vital growth factors like TGFβ, activin A, and extracellular matrices. Scientists developed feeder-free alternatives using Matrigel, which contains laminin, collagen IV, and heparin sulfate proteoglycans. Matrigel supports stem cell growth with defined media formulations and stands as one of the first alternatives to feeder-dependent culture. The mouse sarcoma origin creates risks of xenogeneic contamination.
Scientists developed more defined substrates, including specific laminin isoforms (-511/-521), vitronectin, and E-cadherin . Synthetic polymer-based platforms improved reproducibility with zwitterionic hydrogels like PMEDSAH and aminopropylmethacrylamide (APMAAm). Graphene-based surfaces show promise to maintain pluripotency without extracellular matrix coating.
Growth Factor Protocols for Directed Differentiation
Scientists expose cells to specific growth factors that mirror developmental cues to direct stem cell differentiation. The essential protocols start with either:
- Embryoid body (EB) formation through cell aggregation in suspension culture
- Monolayer differentiation with sequential growth factor exposure
Specific growth factor combinations guide lineage-specific development. Neural differentiation needs bFGF, heparin, and N2 supplement . Ten-day-old EBs naturally develop cardiac differentiation, while Activin A drives endoderm induction. BMP-4, TGF-β1, retinoic acid, and HGF guide cells toward specific fates by activating distinct signaling pathways .
3D Organoid Models for Tissue-Specific Development
Scientists have advanced beyond simple differentiation to create complex 3D organoid models that mirror tissue architecture. Stem cells form organoids through their self-organizing capabilities within supportive 3D matrices . These structures develop more intricate morphology than traditional cultures and provide better tissue development modeling.
Scientists start organoids by embedding stem cells in hydrogels like Matrigel, alginate, or synthetic PEG matrices. Matrix physical properties combined with bioreactors that supply essential nutrients improve organoid development. This method has created models of intestine, brain, kidney, and liver that are a great way to get insights for disease modeling and drug screening .
Results and Discussion: Clinical Applications of Differentiated Stem Cells
Scientists have turned theoretical possibilities of stem cell treatments into real therapies over the last several years. Their rigorous research and clinical trials now help treat conditions that were previously untreatable.
Cardiac Repair Using iPSC-Derived Cardiomyocytes
iPSC-derived cardiomyocytes (iPSC-CMs) show remarkable potential to treat heart failure and myocardial infarction. Transplanting these cells into damaged hearts boosted left ventricular ejection fraction by 8.23% compared to control groups. The cardiac function improved most during 4-8 weeks after treatment, but its effectiveness decreased beyond this period.
Research on non-human primates revealed that combining iPSC-CMs with endothelial cells boosted graft size, blood vessel formation, and heart function after ischemic reperfusion . These improvements came from remuscularization of damaged tissue and better vascularization within grafts .
Neural Regeneration in Parkinson’s Disease Models
iPSC-derived neural stem cells (NSCs) show great promise to treat Parkinson’s disease. These cells can turn into dopaminergic neurons—the main cell type lost in PD. The transplanted cells survive and help through dopamine secretion and trophic support.
Clinical trials proved NSC transplantation safe and showed improved motor function in PD patients. Human iPSC-derived NSCs with SNCA gene knockdown helped mouse models with better coordination, balance, and movement. The mice ended up living longer .
List of Diseases Treated by Stem Cells in Clinical Trials
Clinical trials now use stem cell therapies to treat many conditions:
- Blood disorders: Leukemia, lymphoma, multiple myeloma
- Neurological conditions: Parkinson’s disease, Alzheimer’s, ALS
- Cardiovascular diseases: Heart failure, myocardial infarction
- Immune disorders: Multiple sclerosis, type 1 diabetes
- Other conditions: Osteoarthritis, perianal fistulas in Crohn’s disease
Doctors have successfully used hematopoietic stem cell transplants (bone marrow transplants) for decades, mainly to treat blood-related cancers. Stem cells are a great way to get help when regular treatments don’t work well enough. However, we need more research before widespread use becomes possible.
Conclusion
This piece explores the remarkable trip of stem cells as they change from their undifferentiated state to specialized cells with specific functions. The molecular mechanisms that drive differentiation—from transcription factors like OCT4 and SOX2 to complex epigenetic modifications—show the intricate biological programming that guides cellular development. Scientists proved that adult cells could be reprogrammed back to a pluripotent state through Yamanaka factors, which without doubt revolutionized our understanding. The process still faces challenges with genetic stability.
Scientists have reshaped the scene of stem cell research through advanced culturing techniques. They now work with sophisticated systems and have moved from simple feeder layers to defined substrates. Complex 3D organoid models that better mimic natural tissue environments allow more precise control over differentiation pathways and improved modeling of human diseases.
Clinical applications of differentiated stem cells keep expanding rapidly. These therapies offer hope for conditions that doctors once thought untreatable, from cardiac tissue regeneration to neural cell transplantation for Parkinson’s disease. Many applications remain experimental, but successful treatments like hematopoietic stem cell transplants show their true potential.
The field must overcome several challenges ahead. Scientists need to improve differentiation efficiency, ensure genetic stability, and scale production for clinical use. The science behind cellular reprogramming continues to advance, and stem cell therapies will become key components of modern medicine. These therapies will offer customized treatment options for patients worldwide. The trip from laboratory findings to bedside treatment shows how fundamental biological research can reshape clinical practice and change human lives.